Navigate the regulatory landscape and efficiently drive strategic decisions
-
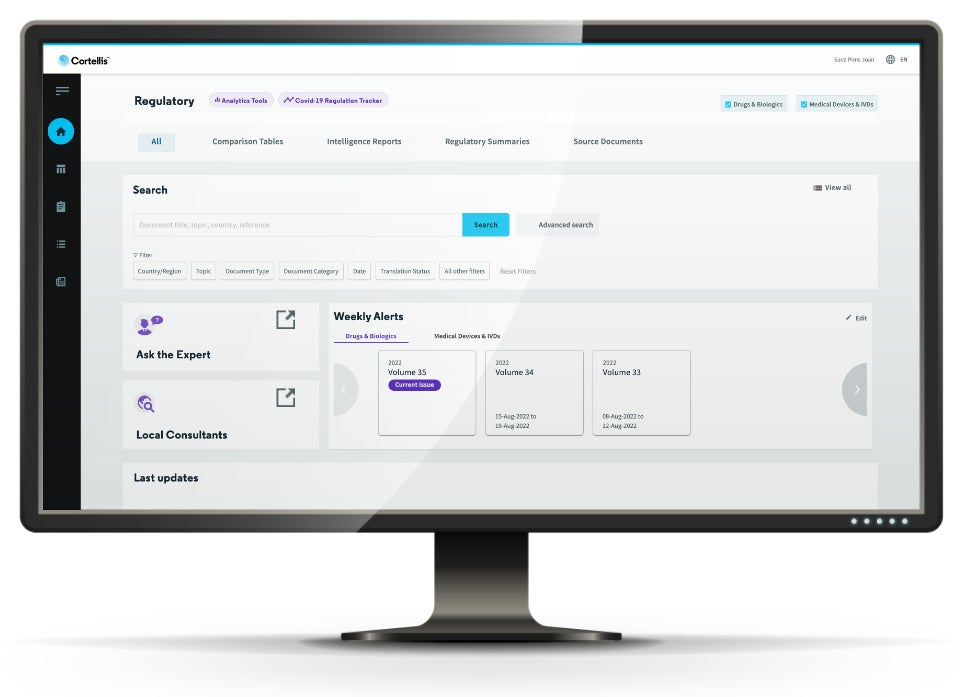
Comprehensive repository of pharma regulatory intelligence
Get full and timely coverage of global health authority requirements on healthcare products such as drugs, biologics, medical devices and IVDs. -
Critical information at your fingertips
Access exclusive expert reports for both in-depth county-based analysis or global comparison on hot topics along the healthcare product development life cycle. -
Specialised customer service ecosystem
Get professional customer support ensured by our competent multilingual subject matter experts, local consultants, and biopharma regulatory consulting services.